Now back to basics I know but its surprising how the simple stuff can catch you out! So lets get this party started:
Electrons - have a negative charge and is equal to that of protons
Protons - have positive charges and can be found as the smaller number under an element.
Neutrons - has a neutral charge and is found out by taking the atomic mass (smaller number) away from the relative atomic mass (the bigger number)
Some atoms are quite happy to stay on their tod without any need of bonding, these are known as 'noble gases'. These elements make up the group 0/8 on the periodic table and this purely means their outer shell is full of electrons so there is no need for any actual bonding to occur. Being that their outer shell is full they are extremely stable elements and almost never react because of this. However, as the group number decreases the less stable an element is, making group 1 very unstable.
There are 3 types of bonding and as you have probably guessed they are ionic, covalent and metallic.
Ionic - occurs between a metal and non-metal where electrons are transferred directly between one element and the other
Covalent - occurs in compounds with two non-metals where electrons are shared between the two elements
Metallic - occurs in metals and the electrons are shared between all the atoms
That's not a lot of info for the stuff that makes up your entire existence, so lets go a little deeper...
Ionic bonding-
As we now know, Ionic bonding occurs between a metal and a non metal which fully transfers electrons from one element to another. This transferal of electrons gives both elements a charge which is either positive or negative. Lets take a look at an ionic bond in action:
Simple covalent lattices are held by weak forces between molecules but the atoms with an individual molecule are strongly bonded together.
Properties of simple covalent lattice -
- Low melting and boiling points due to weak forces
- Don't conduct electricity
- Soluble in non-polar solvents
Giant covalent lattice properties -
- High melting and boiling points
Regular properties -
- Low melting points
- Poor Conductivity
- Not soluble
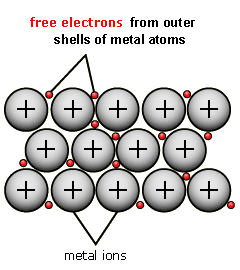
Metallic-
- Strong and requires a lot of energy to break
- High melting and boiling points
- Conducts electricity well due to having free 'delocalized' electrons
The attraction between the metal ions and the delocalized electrons must be broken to melt or boil and because of this metals have a higher melting and boiling point. These delocalized electrons are free to move throughout the entire structure.
Electrons - have a negative charge and is equal to that of protons
Protons - have positive charges and can be found as the smaller number under an element.
Neutrons - has a neutral charge and is found out by taking the atomic mass (smaller number) away from the relative atomic mass (the bigger number)
Some atoms are quite happy to stay on their tod without any need of bonding, these are known as 'noble gases'. These elements make up the group 0/8 on the periodic table and this purely means their outer shell is full of electrons so there is no need for any actual bonding to occur. Being that their outer shell is full they are extremely stable elements and almost never react because of this. However, as the group number decreases the less stable an element is, making group 1 very unstable.
There are 3 types of bonding and as you have probably guessed they are ionic, covalent and metallic.
Ionic - occurs between a metal and non-metal where electrons are transferred directly between one element and the other
Covalent - occurs in compounds with two non-metals where electrons are shared between the two elements
Metallic - occurs in metals and the electrons are shared between all the atoms
That's not a lot of info for the stuff that makes up your entire existence, so lets go a little deeper...
Ionic bonding-
As we now know, Ionic bonding occurs between a metal and a non metal which fully transfers electrons from one element to another. This transferal of electrons gives both elements a charge which is either positive or negative. Lets take a look at an ionic bond in action:
So Na²O has two sodium and one oxygen. Ever wondered why? Well
let me show ya! What do we know about these two elements as separate entities?
Sodium (Na) is in group one and has one electron on its outer shell.
Oxygen (O) has six electrons in its outer shell.
What every element want to do is complete its shell and fill in all of its outer shell to make it full. So with this in mind, we have learned something new that can aid us in this transferal.
Sodium needs to remove one electron from it outer shell to get a full outer shell to become the magical thing known as a 'stable' element.
Alternatively oxygen requires two extra electrons to fill its outer shell to become 'stable'.
As shown by the diagram above, two lots of sodium are needed to give an electron and fill the outer shell of oxygen. To finish this the elements are bracketed separately and given a charge.
Giant ionic lattices -
- Each ion is surrounded by oppositely charged ions
- These ions attract from all directions to form a giant ionic lattice
Properties -
- High melting and boiling points
- Dissolves in polar solvents such as water
- Conduct electricity when in liquid form or aqueous solution
Covalent -
- The negative electrons are attracted to the positive charge of the nuclei, this attraction overcomes the repulsion between the positively charged nuclei
- Occupies the space between two atoms
- Occurs between two non-metals and the electrons are shared
The simplest way to represent a covalent bond is with two hydrogen atoms as both the hydrogen's have 1 electron and require another to fill its outer shell.
As you can see, there is no need for brackets as the electrons are clearly being shared between an overlapped point. This satisfies both hydrogen's need to fill its outer shell. These non-metal covalent bonds can share more than one atom to accommodate to the other needs by having double bonds and triple bonds. Simple covalent lattices are held by weak forces between molecules but the atoms with an individual molecule are strongly bonded together.
Properties of simple covalent lattice -
- Low melting and boiling points due to weak forces
- Don't conduct electricity
- Soluble in non-polar solvents
Giant covalent lattice properties -
- High melting and boiling points
Regular properties -
- Low melting points
- Poor Conductivity
- Not soluble
Metallic-
- Strong and requires a lot of energy to break
- High melting and boiling points
- Conducts electricity well due to having free 'delocalized' electrons
The attraction between the metal ions and the delocalized electrons must be broken to melt or boil and because of this metals have a higher melting and boiling point. These delocalized electrons are free to move throughout the entire structure.
Positive ions remain where they are the only thing that moves is the delocalized electrons and this attraction is very strong giving metallic bonding its properties. A metallic lattice is held by the opposite attraction of the particles.
That's all you need to know about simple bonding!
Did you find this helpful? Feel free to drop a comment and let me know what you liked and if I have missed something!




Comments
Post a Comment